Almost nowhere are labeling requirements more stringent than in the pharmaceuticals industry: serialization, aggregation, tracking & tracing and tamper-evident labels are becoming more and more important for pharmaceutical labeling companies – and mean constant change. Ensuring the highest degree of security in medicine labeling requires the perfect interplay between different technologies – for printing, identification, labeling and control – in the tightest of spaces.

- HERMA applicators and labeling machines have been handling the most challenging tasks at pharmaceutical labeling for decades – with reliability, precision and speed.
- With their standardized modules and sophisticated modular design, HERMA labeling machines always deliver reproducible results – attuned to your individual production conditions.
- In every regard, HERMA gives you the sure footing to meet the requirements of today and tomorrow.
Safety in pharmaceutical labeling
In pharmaceutical production, any deviations in quality have direct effects on the health of people or animals. That is why we place a massive emphasis on maximum reliability in printing, coding and labeling, up to and including the controlled rejection of products or labels. HERMA provides a powerful overall package with that in mind, including standard control systems, serialization, tamper-evident labeling solutions and machine designs in accordance with GMP guidelines with comprehensive qualification.
1. Reliable Control Systems
HERMA pharmaceutical labeling machines equipment is designed to meet the strictest requirements and eradicate operating errors. The aim: to ensure that only products and labels found to be good remain in the production process.
- To cover every customer requirement, the modular design of HERMA systems includes standardized brackets for integrating all kinds of conventional sensors and camera systems.
- The dual control system prevents people from bypassing the system manually.
- The final rejection crosscheck ensures that the product has been successfully removed from the production process.
2. A Variety of Printing Options
Ever-growing requirements make printing variable data on pharmaceutical labels a challenge. Different printing methods may make economic sense depending on the size and content of the printed image, the type of labels used and the production speed.
- The standardized HERMA printer frame lets you integrate any kind of conventional hot foil stamping and thermal transfer printers into our HERMA applicators. When using a large number of applicators, that provides not only economic benefits but also maximum flexibility.
- It gives the user all kinds of freedom, including the ability to convert an existing system for other printer types later.
- Contactless labeling with laser printers is also no issue. The advantage with these printers is that they do not require any consumables and involve very low operating costs. Furthermore, even tiny font sizes can be printed pin-sharp at the highest speeds.
3. Comprehensive Qualification
HERMA pharmaceutical labeling machines are qualified according to the individual wishes and requirements of our customers. That means: you define the specific scope of performance of the machines and the points where you want us to help. A top priority for us in this regard is comprehensive qualification documentation, which we compile in accordance with regulatory standards and are happy to validate with you during machine acceptance testing.
- We coordinate with you to prepare the qualification masterplan and project qualification plan.
- We use a failure mode and effects analysis (FMEA) and a traceability matrix for process validation, for instance, in the hardware and software specifications.
- The key elements in the final stage of the process are installation qualification (IO), operational qualification (OQ), FAT and SAT.
4. Design of our High-Performance Labeling Machines
Whether you need labels with tear-off flaps, hanger labels for infusion bottles or booklet labels: HERMA labeling machines are especially suitable for covering a wide range of products and applications reliably – and for meeting the very specific requirements of pharmaceutical labeling.
- Their modular design lets you change format parts quickly and in most cases without needing tools.
- In terms of line clearance, HERMA machines are optimized to ensure a clean and smooth process sequence.
- The long-term, worldwide and online availability of spare-parts ensures a high level of investment security.
- HERMA labeling machines have a compact design that makes them easy to integrate into existing packing and packaging systems.
- Process and production excellence are crucial to maintaining the high quality of our applicators and labeling machines. Our new production facilities in Filderstadt, Germany are setting new benchmarks in this regard.
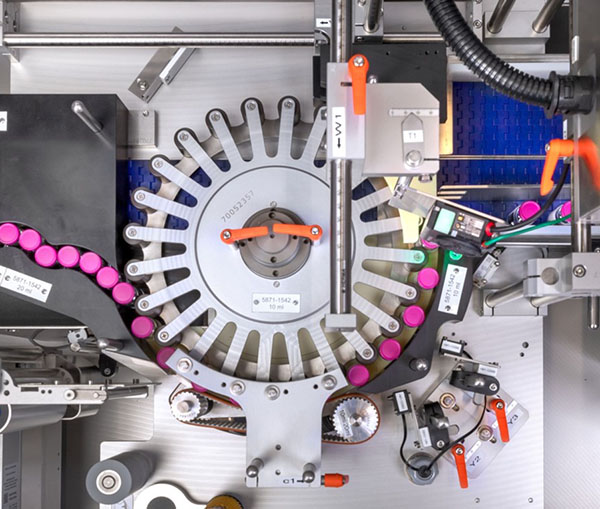
The High Requirements Placed on Pharmaceutical Labeling Solutions
Medication is as lucrative a field of business as ever for counterfeiters. The EU Commission has responded to this threat with the Falsified Medicines Directive 2011/62/EU that came into force on February 9, 2019, and has provided pharmaceuticals companies with strict requirements for identifying and tracing pharmaceuticals and protecting them from manipulation. We have developed some hugely effective solutions for serialization, track and trace, aggregation and tamper-evident labeling.
Serialization
To comply with the Falsified Medicines Directive, all packaging for prescription medication brought to market within the EU must have a unique, individual serial number. The numbers are generated by official organizations, regulated and stored in a database. Similar solutions are being considered or have already been implemented in many other markets.
- Special precautions are required to record the serial numbers or 2D code of rejected labels or packaging. Such labels or packaging must not be made available or should be made available only with checks.
- HERMA labeling machines can be fully equipped to perform serialization tasks in medicine labeling in compliance with EU regulations or other international provisions.
- Aggregation (that is, the aggregation of serialized products) is often necessary or useful in combination with serialization. The HERMA product portfolio includes the perfect printing and applicator systems for this task.
Track & Trace
Where does the product come from? Who produced it? In many markets, the path taken by a drug must be fully traceable at all times – from the manufacturer to the end consumer. Track and trace lets you record its full route along the supply chain in real time. One prerequisite for doing so is that the product can be clearly identified by a serial number. HERMA provides you with powerful printing and labeling solutions for the special requirements involved in track and trace.
Aggregation
Aggregation is the secure combination of individually serialized packages into larger logistical units such as bundles, cardboard boxes or pallets. Unlike the serialization of individual packages, aggregation is not a legal requirement in the EU, although it is in other markets. However, it offers pharmaceutical companies another added benefit: establishing a relationship between the individual products and the logistical units makes it much easier to trace the path of individual medications. That also makes it easier to coordinate recalls or report stolen products, for instance.
- To print the aggregated “group codes” on labels and apply them, HERMA offers particularly reliable print and apply systems for every aggregation level.
Tamper-Evident
The EU and many other markets stipulate that pharmaceutical packaging for medicines such as prescription drugs, for instance, requires a tamper-evident seal as a security feature. If you wish to continue using your current folding boxes, the use of tamper-evident labels is a particularly interesting option.
- HERMA offers a range of efficient and reliable solutions for sealing packaging with seals or tamper-evident labels. Our specially developed tamper-evident labeling machines are already deployed in a large number of markets.
- Where transparent tamper-evident labels are used, the adhesive with luminescent particles can be adjusted to enable a UV sensor on the labeling machine to detect the difference between the folding box and label. This allows you to check the successful application of the tamper-evident labels directly.
- If the tamper-evident labeling is printed on, a camera system or contrast scanner can perform this control function.
- If none of these methods work, gloss sensors can be used to detect any difference between the gloss on the folding box and the gloss on the tamper-evident labeling labels, and therefore determine the presence of the labels.
GMP Standards
Quality assurance plays a central role in the manufacture of medication, with direct effects on patient safety. A quality management system in accordance with GMP (“Good Manufacturing Practice”) helps to ensure product quality and the obligatory fulfillment of healthcare authority requirements.
- HERMA pharmaceutical labeling machines are developed and built in accordance with GMP requirements. This is how they help to achieve the prescribed quality levels in the production processes and environment.
- The provisions can vary from country to country.
- GAMP (“Good Automated Manufacturing Practice”) is another closely associated standard. It includes guidelines (which are not legally binding) for codes and programming machine controls. Its aim is to rule out undesirable functions from the outset.
The Right Labels for Pharmaceutical Products
HERMA labeling machines do not just cover all the conventional kinds of labeling, including wrap-around labeling, top labeling, bottom labeling and two-sided labeling. They also apply special materials like booklet, documentation or hanger labels with precision, speed and smoothness for pharmaceutical labeling.
Product Types
Label Types
HERMA Labeling Machines for the Pharmaceuticals Industry
Reliable, flexible and highly productive: with us, you can find powerful labeling machines that meet your requirements. To name just three examples: the 132M HC provides high-speed wrap-around labeling for free-standing vials, the 362E TE is a particularly economical solution for tamper-evident labeling and the 362M guarantees precise side labeling and the option of wrap-around labeling with an unrivaled precision – with HERMA you are ready for any labeling task.
Finding the Right Pharmaceutical Labeler for You
Determining Your Requirements
You tell us your requirements for your labeling and your production conditions. Using your samples of products and labels as a basis, we develop a solution concept and discuss any unresolved issues with your specialists. If you supply us with a user requirement specification (URS), our experts will check it and provide appropriate comments.
Quotation and Order
After matching your requirements with our solution concept and following any initial machine tests, we prepare a detailed quotation for you to serve as the basis for your order. In the process, we also explain the framework conditions for the order workflow, such as deadlines for the design review, FAT or SAT.
Design Review & Construction
In the design review, we discuss the concept for the labeling machine with you again. The main focus of these discussions is on the drafts for the layout of the machine that have been prepared in the meantime and on automation. We also use tools such as design FMEAs or a traceability matrix. We add the details of the design following approval. This stage ends with the creation of drawings and part lists for the procurement of materials and approval for construction.
Building and Commissioning
As soon as the necessary materials are available, we build the mechanical and electrical systems for the labeling machine. That is followed by commissioning. In this stage, we also connect control systems such as cameras and printers to our control unit. This serves as the basis for running the various product/label combinations as specified in your URS.
Pre-FAT and FAT
Once commissioning is complete, we prepare the final version of the qualification documents. You then receive them for the advance check. We also use them for the internal pre-FAT, in which we perform the tests and service cycles of the later FAT. For the FAT, you come to our facilities and test the performance of the machine based on the qualification documents alongside our experts.
Installation and SAT
Finally, the machine is dispatched and installed in your plant; the SAT, in which acceptance testing is performed on the machine in interaction with the overall production line, is then conducted there.
Find out exactly what the path to your pharmaceutical labeling machine looks like and how our special team is set up to do it here:
"Our sales engineering team works together with you to develop the perfect solution for your perfect labeling application."
Ulrich Fischer
Project Design Engineer
"Project management is our passion: clear and reliable communication, with a high level of personal commitment to your labeling machine. We walk the talk."
Max Kunert
Head of project management & product management
Our promise: A belief-based added value in pharma labeling
Find out here how our values have a lasting impact not only on medicine labeling, but on all our labeling solutions:
Success Stories
Gain an insight into the challenges faced by some of our pharmaceuticals customers and learn how HERMA labeling machines helped to successfully overcome them in our Success Stories section.
Contact
You can contact our team by phone or e-mail if you require any more information about our pharmaceutical labeling solutions or products and services or need our expert advice.
Request for quotation
We produce labeling solutions according to your specifications - suitable for your application!
![[Translate to English:] HERMA Formular Etikettiermaschinen Angebot anfordern](/fileadmin/Etikettierer/kacheln/HERMA_Formular_Etikettiermaschinen_Angebot_anfordern.jpg)
Please take a moment to provide us with some detailed information about your labeling solution requirements on the following page so that we can give you a good consultation.
Find out more
HERMA Labeling Businews
Our industry magazine for labeling solutions
Read our issue # 001 on the subject of pharmaceutical labeling.
















